By Rose Paddock, Class of 2010
Edited and illustrated by Dr. Peg Miller, ADDL pathologist
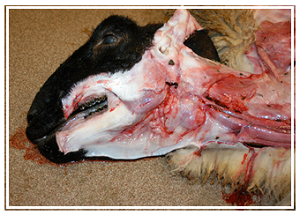
Weanling Suffolk lamb, found dead without observed clinical signs. Note tissue pallor, translucent water blood, and submandibular subcutaneous edema ("bottle jaw").
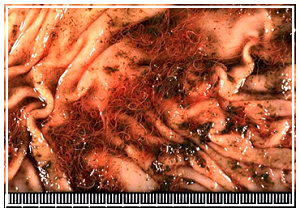
Opened abomasums with pale mucosa, scanty red-brown content, and tangles of the barber-pole worm,
Haemonchus contortus
Haemonchus contortus is the most economically significant parasite of sheep and goats throughout much of the United States and the world, due to the severity of the parasitism and the emerging anthelmintic resistance. Haemonchosis also affects New World camelids. It is a common cause of death in all these species, and often takes the practitioner and owner by surprise, as clinical signs can be subtle. Rapid diagnosis, strategic prevention, and pasture management, along with a thorough understanding of the pathophysiology of haemonchosis facilitates the development of herd health protocols to control this deadly disease.
The life cycle of Haemonchus is simple. Infective L3 larvae, ingested by the host on pasture, develop into adults in the abomasum and produce eggs that are passed in the feces. The eggs hatch in the feces and undergo two molts, becoming L3 larvae that can migrate up blades of grass in drops of moisture. In proper conditions, it takes only 3-4 days for an egg deposited in feces to develop to the L3 stage. Depending on temperature and moisture, L3 larvae can survive on pasture up to 6 months. Adult worms only survive several months in the host.
Haemonchus contortus favors warm moist climates; however, it has adapted successfully to most of the United States. Optimum conditions for H. contortus exist from May through September in Urbana, Illinois; the season is even longer in the Southeast. In the winter, larvae become metabolically inactive, undergoing hypobiosis. Most animals succumb to haemonchosis in the spring, due to the combined assault of larvae emerging from hypobiosis and the immunosuppression of late pregnancy. This results in the periparturient rise in egg shedding, resulting in numerous infective larvae on pasture at the time when young animals are most susceptible. Although all ages of sheep and goats are susceptible to haemonchosis, recently weaned animals are usually the most vulnerable. Besides age, other factors that increase susceptibility include overgrazing, dense stocking rates, and inadequate nutrition, particularly protein intake.
The most common clinical signs are failure to thrive and weight loss. As worm burdens increase, more severe signs, such as anemia, hypoproteinemia, submandibular edema (bottle jaw), weakness, and collapse, may develop. Unlike other gastrointestinal nematodes, H. contortus does not usually cause diarrhea. Due to the nonspecific signs and lack of diarrhea, haemonchosis is often undiagnosed until death. The death can appear sudden, even though the course of infection may have been prolonged.
Clinical pathology findings include anemia, hypoproteinemia, and eosinophilia. In one experimental study, mean packed cell volume dropped to 15% in infected sheep 35 days after infection, and mean eosinophil count increased from <5% to 25% of circulating white blood cells 56 days after experimental infection. Eosinophilia decreased from days 63-77 of the experiment, which may have resulted from localization of eosinophils to the abomasum. A significant correlation between eosinophilia and worm burden has been demonstrated.
Clinical diagnosis of haemonchosis is usually based on clinical signs and fecal flotation results. Clinical observation is perhaps the most useful tool in diagnosing and managing haemonchosis, but requires close examination. Because anemia is the principle clinical problem, mucous membrane pallor is one of the easiest ways to monitor sheep, and has proven effective as a diagnostic approach. The FAMACHA system uses a 5-point scale to gauge ocular mucous membrane color, which correlates with packed cell volume in sheep. Owners and veterinarians must be trained in the system and use the official cards, but the FAMACHA system is practical on-farm, and can be used to select animals for strategic de-worming. The FAMACHA system also has been successfully used in goats and camelids. The importance of regular application of FAMACHA scoring cannot be over-emphasized. Many animals submitted to the ADDL with fatal haemonchosis had recent satisfactory FAMACHA scores.
Although fecal flotation is a valuable diagnostic tool to assess gastrointestinal parasitism, H. contortus eggs cannot be distinguished easily from those of other strongylids. Most pastured sheep have some level of gastrointestinal nematode infection, so the presence of strongylid eggs in feces is expected. Counting eggs per gram of feces is useful in developing treatment and control strategies. The quantitative McMaster’s technique, using a McMaster slide with a chamber beneath a printed grid, allows enumeration of eggs per gram of feces. The McMaster’s technique is easily learned by veterinarians and producers, and can also be used to assess anthelmintic effectiveness by measuring the reduction in fecal egg count 7-14 days after deworming.
Most sheep with clinical haemonchosis have high fecal egg counts, up to 10,000 eggs per gram. However, the prepatent period for H. contortus is 15-21 days in sheep, and peracute infections can result in death before eggs are present in the feces. Also, recent anthelmintic treatment may result in decreased fecal egg counts. Because of the wide variability of fecal egg shedding, pooled samples are not recommended for monitoring herd levels of H. contortus. It is more useful to collect individual samples representing all age groups.
Parasitology laboratories can culture larvae from parasite eggs to speciate the nematode; however, larval culture usually takes 10-14 days. Some laboratories perform in vitro analysis of anthelmintic resistance by exposing hatched larvae to various anthelmintic drugs. At Oregon State University and the University of Georgia, a fluorescein-labeled peanut agglutinin test is used for rapid differentiation of Haemonchus eggs from those of other strongyles; H. contortus eggs fluoresce green due to their affinity for peanut agglutinin. This test has been proven to be reliable, is faster than larval culture, and is expected to become more widely available.
Gross lesions and the presence of characteristic nematodes in the abomasum often provide a diagnosis at necropsy. Animals with haemonchosis have marked pallor of mucous membranes and internal tissues. A characteristic gross lesion is widespread subcutaneous edema. This may be most striking in submandibular soft tissues, producing the so-called “bottle-jaw” (Fig. 1). Edema is concentrated in the submandibular soft tissues because the head is often dependent in grazing animals. Hydrothorax, hydropericardium, and ascites are other sequelae of hypoproteinemia as is edema of the abomasal mucosa. Lymph nodes draining the abomasum may double in weight within five days of infection.
The abomasum has dark red-brown contents with multifocal mucosal hemorrhages and H. contortus adults (Fig. 2). In a freshly dead animal, the worms are often alive and writhing; if the animal has been recently treated with anthelmintics, worms may not be found. The adult worm is approximately 2 cm long; the female has a characteristic “barber-pole” appearance due to the red color of the blood-filled digestive tract against the white reproductive tract. The male is all red and slightly shorter. Both worms have a buccal tooth that can pierce the abomasal mucosa to suck blood.
Common histologic lesions, such as interstitial edema in many organs and centrilobular hepatic necrosis, are sequelae of the hypoproteinemia, anemia, and resultant hypoxia. Abomasitis may be seen with increased numbers of mucosal lymphocytes, eosinophils, and mast cells. The local immune response in the abomasum has been studied for vaccine development, which is of particular interest in the face of increasing anthelmintic resistance. Leukocyte levels in the abomasal mucosa peak 5 days after infection, and then may decrease; however, mast cell numbers remain high. Sheep that are resistant to H. contortus may have more numerous mucosal mast cells. Abomasal lymph nodes undergo rapid lymphocyte (CD4+ T-cell) proliferation. Repeated larval exposure often results in protective immunity, so purified larval antigen has been a target for vaccine development. Because local abomasal mucosal immunity is paramount to protection, it may be difficult to develop a parenteral immunization that is effective against H. contortus.
Control of haemonchosis is beyond the scope of this article. The principles involve pasture management, strategic de-worming, and avoiding anthelmintic resistance by using dewormers that result in >90% fecal egg count reduction. A major problem facing the sheep industry is resistance to macrolide dewormers, such as ivermectin and moxidectin. H. contortus has also been resistant to benzimidazoles (e.g., fenbendazole), tetrahydropyrimidines (e.g., pyrantel), and levamisole. Genetic selection has proven useful in controlling haemonchosis. Certain breeds of sheep, notably Barbados and Gulf Coast Native, and certain familial lines are more resistant to parasites, and this resistance is heritable to a large extent. Resistant sheep have had higher IgE levels in nodal tissue three days following infection. There is evidence of genetic resistance in certain goat breeds, as well. A multi-factorial approach to Haemonchus control is paramount to maintaining animal health and productivity, while diminishing further development of anthelmintic resistance.
References:
- Balic A, Bowles VM et al: Cellular profiles in the abomasal mucosa and lymph node during primary infection with Haemonchus contortus in sheep, Vet Immunol Immunopathol 75: 109-120, 2000.
- Bath GF: Non-pharmaceutical control of endoparasitic infections in sheep, Vet Clin Food Anim 27: 157-162, 2011.
- Jacobs HJ, Ashman K, Meeusen E: Humoral and cellular responses folloing local immunization with a surface antigen of the gastrointestinal parasite Haemonchus contortus, Vet Immunol Immunopathol 48: 323-332, 1995.
- Jurasek ME, Bishop-Stewart JK et al: Modification and further evaluation of a fluorescein-labeled peanut agglutinin test for identification of Haemonchus contortus eggs, Vet Parasit 169:209-213, 2010.
- MacKinnon KM, Mullarky IK, Notter DR: Effects of Haemonchus contortus on the humoral and cellular immune response of parasite-resistant hair sheep, Vet Immunol Immunopathol 128: 288-289, 2009.
- Maxie M. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Philadelphia: Saunders Elsevier, 2007.
- Pugh, D. Sheep and Goat Medicine. Philadelphia; Saunders, 2002.
- Smith, BP. Large Animal Internal Medicine. St. Louis: Mosby Elsevier, 2009.
- Yacob HT, Mistre C et al: Parasitological and clinical responses of lambs experimentally infected with Haemonchus contortus (L3) with and without ivermectin treatment, Vet Parasit 166: 119-123. 2009.
- Zajac A: Gastrointestinal nematodes of small ruminants: lifecycle, anthelmintics, and diagnosis, Vet Clin Food Anim 22: 529-541, 2006.