 |

Search

|
 |
 |
Diagnosis of Porcine Circovirus
Associated Diseases (PCVAD)
in ADDL from infected pigs.
|
 Porcine circovirus (type 1) was discovered in 1972. The
virus had been studied and it was concluded that it is not pathogenic based on
animal inoculation studies and high prevalence in the swine population. Type 2
PCV (PCV2) drew swine veterinarians' attention after the description of
Post-weaning Multisystemic Wasting Syndrome (PMWS) in 1997 or earlier. PCV2 is
thought to be the only consistent essential infectious cause of PMWS since the
level of virus in swine tissues or serum is directly proportional to severity
of clinical disease and lesions. Porcine circovirus (type 1) was discovered in 1972. The
virus had been studied and it was concluded that it is not pathogenic based on
animal inoculation studies and high prevalence in the swine population. Type 2
PCV (PCV2) drew swine veterinarians' attention after the description of
Post-weaning Multisystemic Wasting Syndrome (PMWS) in 1997 or earlier. PCV2 is
thought to be the only consistent essential infectious cause of PMWS since the
level of virus in swine tissues or serum is directly proportional to severity
of clinical disease and lesions. |
Even though the prevalence of PCV2 infection
is very high and can be 95-100% of the pig population, the incidence of PMWS is
typically low, ranging from 5-15% of pigs on affected farms. Although PCV2 is
thought to be the only essential infectious agent, PMWS has been difficult to reproduce
in pigs inoculated only with PCV2. In a majority of studies, PCV2 alone as
inoculum failed to reproduce any pathological changes and/or clinical signs similar
to those observed in field cases of PMWS, even though the virus or viral
antigens were detected in various tissues. In a few studies, a low proportion
of pigs developed some lesions or clinical signs of PMWS, but few developed all clinical signs and histologic lesions of PMWS, although the virus or viral
antigens were detected in various tissues. Recently, new terminology was
introduced and approved by the American Association of Swine Veterinarians
(AASV) - Porcine Circovirus ASSOCIATED disease (PCVAD)- used to denote the
entire spectrum of diseases that are associated with PCV2 including PMWS,
respiratory disease, reproductive failure, diarrheal disease, Porcine Dermatitis
Nephritis Syndrome (PDNS), and high mortality in pigs. In many cases,
different co-factors (infectious and non-infectious) are required, along with
PCV2, to reproduce clinical signs and lesions in these pigs. The following pathogens
should be considered as differentials when diagnosing PCVAD in a swine population:
Porcine Respiratory and Reproductive Syndrome (PRRS), Swine influenza virus
(SIV), Transmissible Gastroenteritis/Porcine Respiratory Corona-virus
(TGE/PRCV), Porcine Parvovirus (PPV), pestiviruses, M. hyopneumonia, and Salmonella. Also, one should take into consideration the history of the
animals' on- farm management, vaccination, breed, stress, etc. PCV2 is
infectious to swine, can replicate at a high level in sick animals, is
associated with unique lesions and has a significant impact on the swine
industry.
Detection of porcine circovirus types1 and 2, as well as
detecting antibodies to PCV, can be accomplished using several testing methods. |
 |
 |
Virus isolation of PCV |
Virology Section: Fluorescent
antibody on tissue sections (FATS), electron microscopy (EM) and isolation of
the virus from infected tissues (VI). The best samples for virology assays are
fresh tissues (lung, tonsil, enlarged lymph nodes, kidney, spleen) submitted on
ice packs. |
| |
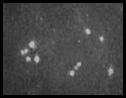
 |
Detection of PCV using Electron Microscopy |
| |
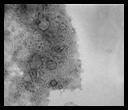
 |
Detection of PCV by FATS |
 |
 |
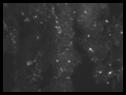
IFA assay using serum samples with antibody to PCV |
Serology
Section: Antibodies to porcine circovirus can
be detected in the Serology Section by IFA. Submit clear serum. |
 |
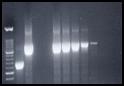 |
Detection and typing of PCV1 and PCV2 virus by PCR in serum
and tissues. |
Molecular Diagnostics:Porcine circovirus can be detected and typed in
infected tissues or serum samples by PCR. |
 |
 |
Detection of the PCV virus in tissues by IHC |
Histopathology section:PCV virus can be detected by Immunohistochemistry
(IHC) in infected tissues. |
 |
Detection of other pathogens is also possible in ADDL upon
request. Cost of tests and submission information can be obtained on ADDL's
website - www.addl.purdue.edu - or by
calling (765) 494-7440.
Confirmation of a diagnosis of PCVAD requires the
demonstration of PCV2 in high concentrations within typical lesions.
For necropsy examination at ADDL, submit affected live
pigs or pigs that have recently died.
If necropsy is performed onsite, collect serum and the
following tissues: several enlarged lymph nodes, tonsil, spleen, kidney, liver,
lung (several locations that are representative of all gross lesions observed)
and ileum. Fix one set of tissues in 10% neutral buffered formalin. Send
additional sets of fresh, chilled tissues (1 set each for virology and
bacteriology if desired) placed in separate sterile, sealable bags. When
TGE/coronavirus is suspected as a co-factor, also include jejunum and a fecal
sample.
For fixation, parenchymal tissues should be no thicker
than 0.5 inches and gut segments approximately 1 inch long and rinsed free of
contents prior to placing the tissues in 10% neutral buffered formalin.
Do not mix lung samples or gut samples with any other
tissue in a specimen bag if submitting for viral or bacterial testing.
Containers should be clearly labeled and all should be
shipped chilled to ADDL by 24 hour couriers. Please do not ship on Friday to
avoid degeneration of tissues in a shipping warehouse over the week-end.
-by Dr. Roman Pogranichniy, Head of Virology/Serology and
Dr. Greg Stevenson, Head of Pathology
References:
-
Allan, GM, Kennedy, S, McNeilly F, Foster JC, Ellis JA,
Krakowka, SJ, Meehan BM and Adair, BM: 1999. Experimental reproduction of
severe wasting disease by coinfection of pigs with porcine circovirus and
porcine parvovirus. J Comp Pathol 121:1-11.
-
Allan GM, McNeilly F, Kennedy S, Daft B, Clarke EG,
Ellis JA, Haines DM, Meehan BM, Adair, BM: 1998. Isolation of porcine
circovirus-like viruses from pigs with a wasting disease in the USA and Europe. J Vet Diagn Invest 1;: 3-10.
-
Clark EG: 1997. Post-weaning multisystemic wasting
syndrome. Proc AASP Annual Meeting. Pp 499-501.
-
Ellis J, Hassard L, Clark E, Harding J, Allan G, Willson
P, Strokappe J, Martin K, McNeilly F, Meehan B, Todd D, Haines D: 1998.
Isolation of circovirus from lesions of pigs with postweaning multisystemic
wasting syndrome. Can Vet J39: 44-51
-
Harding J: 1997. Post-weaning multisystemic wasting
syndrome (PMWS): Preliminary epidemiology and clinical presentation. Proc AASP
Ann Meet p. 503.
Harding JS, Clark EG: 1997. Recognition and diagnosing
postweaning multisystemic wastingsyndrome (PMWS). Swine Health and Production 5:201-203. -
Harms PA, Sorden SD, Halibur PG, Bolin SR, Lager KM, Morozov
I, Paul PS: Experimental reproduction of severe disease in CD/CD pigs
concurrently infected with type 2 porcine circovirus and porcine reproductive
and respiratory syndrome virus. Vet Pathol 38: 528-539.
-
Kennedy S, Moffett D, McNeilly F, Meehan B, Ellis J,
Krakowka S and Allan GM: 2000. Reproduction of lesions of postweaning
multisystemic wasting syndrome by infection of conventional pigs with porcine
circovirus type 2 alone or in combination with porcine parvovirus. J Comp
Pathol 122:9-24.
-
Krakowka S, Ellis JA, Meehan B, Kennedy S, McNeilly F,
Allan G: 2000. Viral wasting syndrome of swine: experimental reproduction of
postweaning multisystemic wasting syndrome in gnotobiotic swine by coinfection
with porcine circovirus 2 and porcine parvovirus. Vet Pathol 37: 254-263.
-
Opriessnig T, Thacker EL, Yu S, Fenaux M, Meng XJ,
Halbur PG: 2004. Experimental reproduction of postweaning multisystemic
wasting syndrome in pigs by dual infection with Mycoplasma hyopneumoniae and porcine circovirus type 2. Vet Pathol 41: 624-640.
-
Pogranichniy RM, Yoon K-J, Harms PA, Sorden S, Daniels
J: 2002. Case-control study on the association of porcine circovirus type 2
and other swine viral pathogens with postweaning multisystemic wasting
syndrome. 14(6):449-456.
-
Pogranichniy RM, Yoon K-J, harms PA, Swenson SL,
Zimmerman JJ, Sorden SD: 2000. Characterization of immune response of young
pigs to porcine circovirus type 2 infection. Viral Immunol 13: 143-153.
-
Sorden SD: 2000. Update on porcine circovirus and
postweaning multisystemic wasting syndrome (PMWS). Swine Health and Production
8:133-136.
-
Tischer K, Bode L, Peters D, Pociuli S, Germann B:
1995. Distribution of antibodies to porcine circovirus in swine populations of
different breeding farms. Arch Virol 140:737-743.
-
Tischer I, Gelderblom H, Vettermann W, Koch MA: 1982.
A very small porcine virus with circular single-stranded DNA. Nature
295:64-66.
-
Rischer I, Mields W, Wolff D, Vagt M, Griem W: 1986.
Studies on epidemiology and pathogenicity of porcine circovirus. Arch Virol
91: 271-276.
-
Rischer I, Rasch R, Tochtermann G: 1974.
Characterization of papovavirus-and picorna-virus-like particles in permanent
pig kidney cell lines. Zentralbl Bakteriol (Origin A) 226:153-167.
|
|
 |

|
 |