 |

Search

|
 |
 |
Laboratory Diagnosis of Bovine Abortion
|
 |
| Middle-trimester Holstein fetus. Note the amniotic plaques that are prominent at this gestational stage. Because the bovine fetus seldom has diagnostic gross lesions, histologic and ancillary testing on fetal and placental tissues is integral to determining the cause for abortion. |
|
Fetal
loss has a major impact on the dairy and beef industries. An estimated 6-10%
of pregnancies terminate in abortion, stillbirth or perinatal death at a
projected cost up to $900 per case.
Diagnosticians at the Indiana Animal Disease Diagnostic
Laboratory (ADDL) and the Heeke ADDL at the Southern Indiana Purdue Agric-ultural
Center (SIPAC) are cooperating in a project to monitor bovine fetal loss in
Indiana and to improve diagnostic success in cases of abortion or perinatal
death. We hope to increase the number and quality of case submissions, improve
ancillary |
methods to identify infectious agents, and correlate pathologic
findings with ancillary test results for meaningful and accurate diagnosis.
Prompt submission of the entire fetus with placenta in every a
bortion case is
ideal. When this is impossible or impractical, please contact the Purdue ADDL
or Heeke ADDL at SIPAC to discuss specimen selection and submission. Necropsy
in abortion cases is performed according to a standard procedure. Macroscopic
examination is used to detect malformations or gross lesions. Selected
formalin-fixed tissues (placenta, brain, heart, lung, liver, kidney, spleen,
digestive tract, and skeletal muscle) are examined histologically to detect
microorganisms, specific lesions, or inflammation as a clue to infectious
disease, or degenerative changes that might incriminate a toxic or nutritional
disease. Ancillary tests are applied as indicated by history or lesions.
Microbiologic tests are applied as routine abortion screens for those cases in
which neither the history nor pathologic examination incriminates a specific
cause. Placenta, lung, liver and abomasal content are appropriate specimens
for bacterial culture; for virologic testing, placenta, lung and spleen. Fetal
pericardial or thoracic fluid can be evaluated serologically to detect
antibodies to known abortifacient agents. Selenium concentrations can be
measured in fetal liver. |
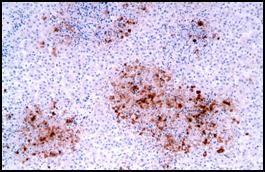 |
| Photomicrograph (courtesy of Dr. Ramos) from a case of
IBR abortion. Necrotic foci in this fetal liver are highlighted by immunohistochemistry for bovine herpesvirus 1. |
|
In 2008,
an infectious cause was suspected in 21 of 47 cases of bovine abortion,
stillbirth, or perinatal death that were examined by necropsy. Twelve of these
cases were attributed to various bacterial pathogens; 6 were considered viral
abortions; 3 cases were attributed to Neosporum caninum. Four of the
six viral abortions were diagnosed as Infectious Bovine Rhinotracheitis (IBR); immunohistochemistry
provides an excellent means to detect IBR herpesviral antigen within the
necrotic foci that are
the diagnostic lesion in this disease. |
Twinning and dystocia were
noninfectious factors that contributed to
several perinatal deaths. Disappointingly,
no cause for abortion,
stillbirth, or perinatal death was determined in 17 cases (36%). Incomplete
submission (particularly omission of the placenta) and post mortem
decomposition of the fetus may account for failure to determine the cause for
abortion or stillbirth in many cases.
In cases of bovine abortion, stillbirth or perinatal death, rapid and accurate
diagnosis is essential for management and reduction of fetal loss. Continued
surveillance and improved diagnostic testing are our goals. The ADDL and Heeke
ADDL at SIPAC hope to increase diagnostic success in bovine abortion cases by
increasing the number and quality of submissions.
Please try to deliver the entire fetus with placenta to the diagnostic
laboratory in each case of bovine abortion.
-by
Dr. Peg Miller, ADDL Pathologist
References:
-
Anderson ML, 2007: Infectious causes of bovine abortion during mid- to
late-gestation. Theriogenology 68: 474-486.
-
Buxton D, McAllister MM, Dubey JP, 2002: The comparative pathogenesis of
neosporosis. Trends Parasitol 18: 546-52.
-
Grooms DL, 2004: Reproductive consequences of infection with bovine viral
diarrhea virus. Vet Clin North Am Food Anim Pract 20:5-19.
|
|
 |

|
 |